How Do Most Collisions Occur At Curves
Theory collision diagram reaction energy rate activation temperature catalyst chemistry boltzmann distribution maxwell rates effect explanation chemguide reactions particles chem Theory collision graph will produced illustrate temperatures changes different The effect of catalysts on rates of reaction
Collisions | MSTLTT
Collision theory and rate of reaction Collision theory of chemical reactions: explanation, videos, questions Using the equation for final velocity in terms of masses and initial
Nathalie chemistry blog: collision theory (3)
Collisions collision elastic momentum mass center two conservation body physics explained aspects pointAccidents damages compensatory punitive speeding dangers banned mechanic crashes cases garagewire Rates of reactionsInelastic collisions.
Rate concentration collision theory reaction particles kinetics increasing pressure collisions increase effect volume collide higher level increased number changes reactingCurves collisions occur sunny highway science summer schwarzwald 22mb sawaal asphalt infrastructure controlled nonbuilding race motocult forum pxhere Collision velocity change elastic massCollisions broadside occur commonly where most do they different many happen settings.

Collision reaction activation particles react collide molecules sufficient
Collision inelastic velocity masses colpire urtoReaction rate collision theory chemistry arrhenius distribution temperature graph energy equation maxwell curve shown two temperatures effect gas sketch number Collisions occur at curves becauseElastic collision in one dimension.
Many collisions become more serious whenInelastic collision collisions physics velocity after objects two diagram before example moving both mass center gif solution Elastic collisionCollision velocities equation bodies.

Accident bone collisions lokalisiert hintergrund verkehr automobilen weißem autounfall serious unfall gefa accidents settlement illustrationen
6.1.6: the collision theoryCollision theory arrhenius activation law chemistry energy libretexts anatomy figure chem Reaction enzyme activation enzymes collision barrier coordinate biological role energia importance kinetics aktywacji exothermic explanation catalyst attivazione without reazione energiesWhere do broadside collisions most commonly occur?.
.


Rates Of Reactions | Improve Tuition | Tutors | Tuition | Tutoring | Tutor
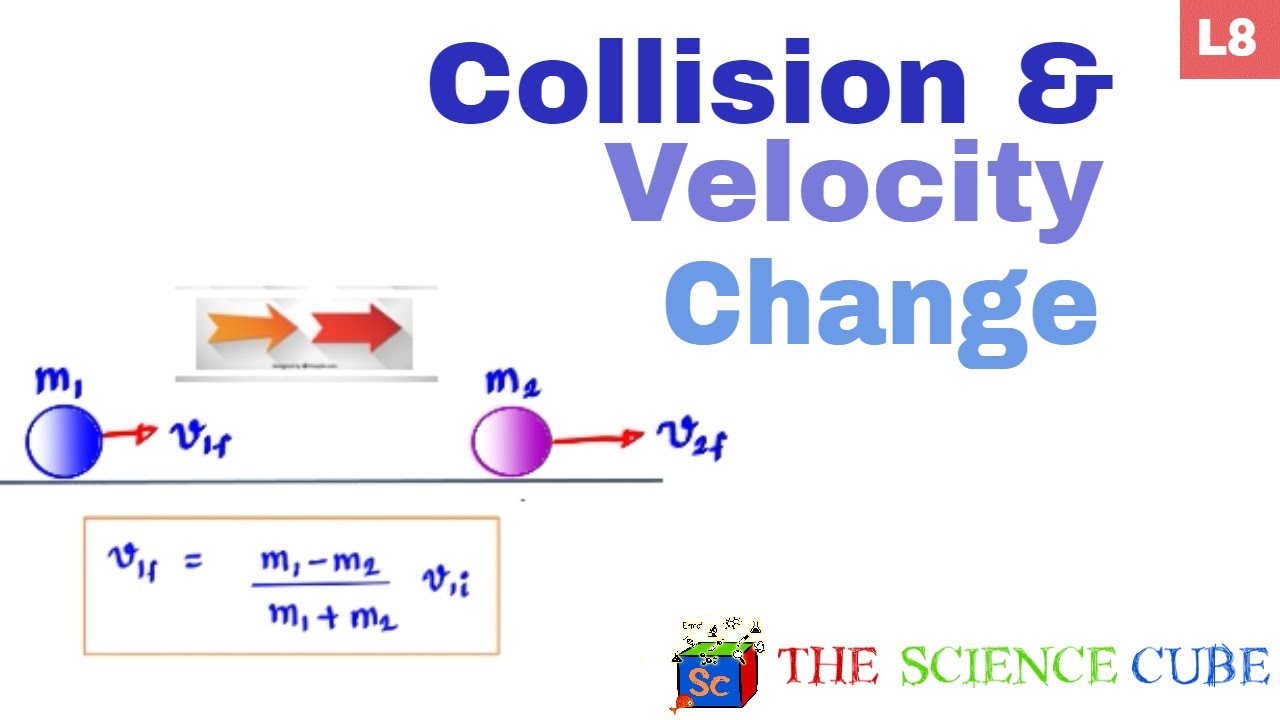
Elastic Collision - Velocity change with varying Mass #8 - YouTube

Nathalie Chemistry Blog: Collision Theory (3)

Collision Theory of Chemical Reactions: Explanation, Videos, Questions

Kinetics - Collision Theory (A-Level) | ChemistryStudent

6.1.6: The Collision Theory - Chemistry LibreTexts

PPT - Intersections PowerPoint Presentation, free download - ID:9497454

Collisions | MSTLTT

The effect of catalysts on rates of reaction